A nuclear reaction is different from a chemical reaction. In a chemical reaction, atoms of the reactants combine by a rearrangement of extranuclear electrons but the nuclei of the atoms remain unchanged. In a nuclear reaction, on the other hand, it is the nucleus of the atom which is involved. The number of protons or neutrons in the nucleus changes to form a new element. A study of the nuclear changes in atoms is termed Nuclear Chemistry.
RADIOACTIVITY
A number of elements such as uranium and radium are unstable. Their atomic nucleus breaks of its own accord to form a smaller atomic nucleus of another element. The protons and neutrons in the unstable nucleus regroup to give the new nucleus. This causes the release of excess particles and energy from the original nucleus, which we call radiation. The elements whose atomic nucleus emits radiation are said to be radioactive. The spontaneous breaking down of the unstable atoms is termed radioactive disintegration or radioactive decay. The disintegration or decay of unstable atoms accompanied by emission of radiation is called Radioactivity.
TYPES OF RADIATIONS
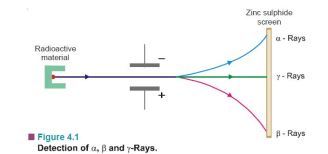
The radioactive radiations are of three types. These were sorted out by Rutherford (1902) by passing them between two oppositely charged plates (Fig. 4.1). The one bending towards the negative plate carried positive charge and were named α (alpha) rays. Those bending towards the positive plate and carrying negative charge were called β (beta) rays. The third type of radiation, being uncharged, passed straight through the electric field and were named γ (gamma) rays. α, β and γ rays could be easily detected as they cause luminescence on the zinc sulphide screen placed in their path.
PROPERTIES OF RADIATIONS
Alpha (α), beta (β) and gamma (γ) rays differ from each other in nature and properties. There chief properties are : (a) Velocity; (b) Penetrating power; (c) Ionisation.
ALPHA RAYS
(1) Nature. They consist of streams of α-particles. By measurement of their e/m, Rutherford showed that they have a mass of 4 amu and charge of +2. They are helium nuclei and may be represented as 42 α or 42 He .
(2) Velocity. α-particles are ejected from radioactive nuclei with very high velocity, about one-tenth that of light.
(3) Penetrating power. Because of their charge and relatively large size, α-particles have very little power of penetration through matter. They are stopped by a sheet of paper, 0.01 mm thick aluminium foil or a few centimetres of air.
(4) Ionisation. They cause intense ionisation of a gas through which they pass. On account of their high velocity and attraction for electrons, α-particles break away electrons from gas molecules and convert them to positive ions.
BETA RAYS
(1) Nature. They are streams of β-particles emitted by the nucleus. From their deflection electric and magnetic fields, Becquerel showed that β-particles are identical with electrons. They have very small mass (1/1827 amu) and charge of – 1. A β-particle is symbolized as 0
–1β or 0
–1e.
(2) Velocity. They travel about 10 times faster than α-particles. Their velocity is about the same as of light.
(3) Penetrating power. β-Particles are 100 times more penetrating in comparison to α-particles. This is so because they have higher velocity and negligible mass. β-particles can be stopped by about 1 cm thick sheet of aluminium or 1 m of air.
(4) Ionisation. The ionisation produced by β-particles in a gas is about one-hundredth of that of α-particles. Though the velocity of β-particles is higher but the mass being smaller, their kinetic energy is much less than α-particles. Hence they are poor ionisers.
GAMMA RAYS
(1) Nature. Unlike α and β-rays, they do not consist of particles of matter. γ-Rays are a form of electromagnetic radiation of shorter wavelength than X-rays. They could be thought of as high-energy photons released by the nucleus during α- or β-emissions. They have no mass or charge and may be symbolized as 00 γ .
(2) Velocity. Like all forms of electromagnetic radiation, γ-rays travel with the velocity of light.
(3) Ionising power. Their ionising power is very weak in comparison to α- and β-particles. A γ-photon displaces an electron of the gas molecule to yield a positive ion. Since the chances of photon-electron collisions are small, γ-rays are weak ionisers.
(4) Penetrating power. Because of their high velocity and non-material nature, γ-rays are most penetrating. They cannot be stopped even by a 5 cm thick sheet of lead or several metres thick layer of concrete.
TYPES OF RADIOACTIVE DECAY
According to the theory put forward by Rutherford and Soddy (1903), radioactivity is a nuclear property. The nucleus of a radioactive atom is unstable. It undergoes decay or disintegration by spontaneous emission of an α- or β-particle. This results in the change of proton-neutron composition of the nucleus to form a more stable nucleus. The original nucleus is called the parent nucleus and the product is called the daughter nucleus.
As evident from above, there are two chief types of decay:
(1) α-decay (2) β-decay
α-Decay
When a radioactive nucleus decays by the emission of an α-particle (α-emission) from the nucleus, the process is termed α-decay. An alpha particle has four units of atomic mass and two units of positive charge. If Z be the atomic number and M the atomic mass of the parent nucleus, the daughter nucleus will have
atomic mass = M – 4
atomic number = Z – 2
Thus an α-emission reduces the atomic mass by 4 and atomic number by 2. For example, Radium decays by α-emission to form a new element Radon,
226 4 222
88 Ra – 2α = 86 Rn
(parent) (daughter)
β-Decay
When a radioactive nucleus decays by β-particle emission (β-emission), it is called β-decay.
A free β-particle or electron does not exist as such in the nucleus. It is produced by the conversion of a neutron to a proton at the moment of emission. Neutron p + e
This results in the increase of one positive charge on the nucleus. The loss of a β-particle from the nucleus does not alter its atomic mass. For a parent nucleus with atomic mass M and atomic number Z, the daughter nucleus will have
atomic mass = M
atomic number = Z + 1
Thus a β-emission increases the atomic number by 1 with no change in atomic mass. An example of β-decay is the conversion of lead-214 to bismuth-214,
214 0 214
82 Pb – –1β = 83Bi
(parent) (daughter)
It is noteworthy that a β-emission results in the production of an isobar. Thus, 214
82 Pb and 214
83 Bi
are isobaric as they have the same mass number 214 but different atomic numbers (82 and 83). One α-emission and two β-emissions yield an isotope. Let us consider the following series of changes.
218 214 214 214
84 Po 82 Pb 83 Bi 84 Po ⎯α⎯→ ⎯β⎯→ ⎯β⎯→
(parent) (isotope)
The parent element 218 84 Po emits an α-particle and subsequently two β-particles, resulting in the formation of 214
84 Po which is an isotope of the parent. Both the parent and the end-product have the same atomic number 84 but different mass numbers (218 and 214).
THE GROUP DISPLACEMENT LAW
The position number of an element in a Group of the Periodic Table corresponds to its atomic number. If the atomic number of a given element is changed, its Group also changes accordingly. We know that an α-emission decreases the atomic number of the parent element by 2 and a β-emission increases the atomic number by 1. Thus : in an α-emission, the parent element will be displaced to a Group two places to the left and in a β-emission, it will be displaced to a Group one place to the right. This is called the Group Displacement Law. It was first stated by Fajans and Soddy (1913) and is often named after them as ‘Fajans-Soddy Group Displacement Law’.
PROPERTIES OF RADIATIONS
Alpha (α), beta (β) and gamma (γ) rays differ from each other in nature and properties. There chief properties are : (a) Velocity; (b) Penetrating power; (c) Ionisation.
ALPHA RAYS
(1) Nature. They consist of streams of α-particles. By measurement of their e/m, Rutherford showed that they have a mass of 4 amu and charge of +2. They are helium nuclei and may be represented as 42 α or 42 He .
(2) Velocity. α-particles are ejected from radioactive nuclei with very high velocity, about one-tenth that of light.
(3) Penetrating power. Because of their charge and relatively large size, α-particles have very little power of penetration through matter. They are stopped by a sheet of paper, 0.01 mm thick aluminium foil or a few centimetres of air.
(4) Ionisation. They cause intense ionisation of a gas through which they pass. On account of their high velocity and attraction for electrons, α-particles break away electrons from gas molecules and convert them to positive ions.
BETA RAYS
(1) Nature. They are streams of β-particles emitted by the nucleus. From their deflection electric and magnetic fields, Becquerel showed that β-particles are identical with electrons. They have very small mass (1/1827 amu) and charge of – 1. A β-particle is symbolized as 0
–1β or 0
–1e.
(2) Velocity. They travel about 10 times faster than α-particles. Their velocity is about the same as of light.
(3) Penetrating power. β-Particles are 100 times more penetrating in comparison to α-particles. This is so because they have higher velocity and negligible mass. β-particles can be stopped by about 1 cm thick sheet of aluminium or 1 m of air.
(4) Ionisation. The ionisation produced by β-particles in a gas is about one-hundredth of that of α-particles. Though the velocity of β-particles is higher but the mass being smaller, their kinetic energy is much less than α-particles. Hence they are poor ionisers.
GAMMA RAYS
(1) Nature. Unlike α and β-rays, they do not consist of particles of matter. γ-Rays are a form of electromagnetic radiation of shorter wavelength than X-rays. They could be thought of as high-energy photons released by the nucleus during α- or β-emissions. They have no mass or charge and may be symbolized as 00 γ .
(2) Velocity. Like all forms of electromagnetic radiation, γ-rays travel with the velocity of light.
(3) Ionising power. Their ionising power is very weak in comparison to α- and β-particles. A γ-photon displaces an electron of the gas molecule to yield a positive ion. Since the chances of photon-electron collisions are small, γ-rays are weak ionisers.
(4) Penetrating power. Because of their high velocity and non-material nature, γ-rays are most penetrating. They cannot be stopped even by a 5 cm thick sheet of lead or several metres thick layer of concrete.
TYPES OF RADIOACTIVE DECAY
According to the theory put forward by Rutherford and Soddy (1903), radioactivity is a nuclear property. The nucleus of a radioactive atom is unstable. It undergoes decay or disintegration by spontaneous emission of an α- or β-particle. This results in the change of proton-neutron composition of the nucleus to form a more stable nucleus. The original nucleus is called the parent nucleus and the product is called the daughter nucleus.
As evident from above, there are two chief types of decay:
(1) α-decay (2) β-decay
α-Decay
When a radioactive nucleus decays by the emission of an α-particle (α-emission) from the nucleus, the process is termed α-decay. An alpha particle has four units of atomic mass and two units of positive charge. If Z be the atomic number and M the atomic mass of the parent nucleus, the daughter nucleus will have
atomic mass = M – 4
atomic number = Z – 2
Thus an α-emission reduces the atomic mass by 4 and atomic number by 2. For example, Radium decays by α-emission to form a new element Radon,
226 4 222
88 Ra – 2α = 86 Rn
(parent) (daughter)
β-Decay
When a radioactive nucleus decays by β-particle emission (β-emission), it is called β-decay. A free β-particle or electron does not exist as such in the nucleus. It is produced by the conversion of a neutron to a proton at the moment of emission. Neutron p + e This results in the increase of one positive charge on the nucleus. The loss of a β-particle from the nucleus does not alter its atomic mass. For a parent nucleus with atomic mass M and atomic number Z, the daughter nucleus will have
atomic mass = M
atomic number = Z + 1
Thus a β-emission increases the atomic number by 1 with no change in atomic mass. An example of β-decay is the conversion of lead-214 to bismuth-214,
214 0 214
82 Pb – –1β = 83Bi
(parent) (daughter)
It is noteworthy that a β-emission results in the production of an isobar. Thus, 214 82 Pb and 214 83 Bi are isobaric as they have the same mass number 214 but different atomic numbers (82 and 83). One α-emission and two β-emissions yield an isotope. Let us consider the following series of changes.
218 214 214 214
84 Po 82 Pb 83 Bi 84 Po ⎯α⎯→ ⎯β⎯→ ⎯β⎯→
(parent) (isotope)
The parent element 218
84 Po emits an α-particle and subsequently two β-particles, resulting in the formation of 214
84 Po which is an isotope of the parent. Both the parent and the end-product have the same atomic number 84 but different mass numbers (218 and 214).
THE GROUP DISPLACEMENT LAW
The position number of an element in a Group of the Periodic Table corresponds to its atomic number. If the atomic number of a given element is changed, its Group also changes accordingly. We know that an α-emission decreases the atomic number of the parent element by 2 and a β-emission increases the atomic number by 1. Thus : in an α-emission, the parent element will be displaced to a Group two places to the left and in a β-emission, it will be displaced to a Group one place to the right. This is called the Group Displacement Law. It was first stated by Fajans and Soddy (1913) and is often named after them as ‘Fajans-Soddy Group Displacement Law’.
Reference: http://bpharmstudy.blogspot.in/2014/06/nuclear-chemistry.html
(function(i,s,o,g,r,a,m){i[‘GoogleAnalyticsObject’]=r;i[r]=i[r]||function(){
(i[r].q=i[r].q||[]).push(arguments)},i[r].l=1*new Date();a=s.createElement(o),
m=s.getElementsByTagName(o)[0];a.async=1;a.src=g;m.parentNode.insertBefore(a,m)
})(window,document,’script’,’//www.google-analytics.com/analytics.js’,’ga’);
ga(‘create’, ‘UA-51872353-1’, ‘wordpress.com’);
ga(‘send’, ‘pageview’);




Reblogged this on jkmhoffman.